how many valence electrons does barium have|Barium : Pilipinas Find the valence of barium and other elements in this table of element . The NecroMerger's bedroom has been sent back to the prehistoric ages - and there's a T-Rex in his bed. Do you shut the door? No, you raise an army of dinosaurs to wake that bed-hogger up. . Honkai: Star Rail is an all-new strategy-RPG title in the Honkai series that takes players on a cosmic adventure across the stars. Hop aboard the Astral .Comedienne Ethel Booba on Tuesday released a vlog sharing the story behind the sudden closure of the Twitter account bearing her name.. Ethel recently disowned the account allegedly due to its politically-charged posts despite having 1.6 million followers. In her latest vlog titled “The Revelation,” the television personality claimed that .
PH0 · Valences of the Elements Chemistry Table
PH1 · Valence Electrons Chart for All Elements
PH2 · How to Find the Valence Electrons for Barium (Ba)
PH3 · Determine valence electrons using the periodic table
PH4 · Complete Electron Configuration for Barium (Ba, Ba2+ ion)
PH5 · Complete Electron Configuration for Barium (Ba, Ba2
PH6 · Barium valence electrons
PH7 · Barium Valence Electrons (And How to Find them?)
PH8 · Barium Electron Configuration (Ba) with Orbital Diagram
PH9 · Barium (Ba)
PH10 · Barium
SpinMyBonus.com lists below 364 casinos that offer a Free Spins Welcome Bonus on sign up, No Deposit Required!This is your chance to play your favorite real money slots for free, and keep your winnings!Some of these are free spins on card registration, which means that you will have to enter your credit or debit card information before actually claiming .
how many valence electrons does barium have*******Mar 23, 2023 There are two ways to find the number of valence electrons in Barium (Ba). The first is to use the Periodic Table to figure out how many electrons Barium has.Barium Find the valence of barium and other elements in this table of element .how many valence electrons does barium have Barium Find the valence of barium and other elements in this table of element . Learn how to write the complete electron configuration of barium (Ba) through orbitals and orbitals. Find out the number of valence electrons, the atomic number, and the electron holding capacity of each .
Discovery date. 1808. Discovered by. Humphry Davy. Origin of the name. The name comes from the Greek 'barys', meaning heavy. Allotropes. Barium. 56. 137.327. Glossary. .December 1, 2021 Leave a Comment. Learn the Barium electron configuration here and get to explore this chemical element from a close dimension. We are going to cover up the electron configuration and the . Watch on. Barium in Periodic table. Barium element is in group 2 and period 6 of the Periodic table. Barium is the s-block element and it belongs to alkaline .
Learn how to find the valence electrons of barium using two methods: periodic table and electron configuration. Barium has 2 valence electrons in the 6s subshell.
how many valence electrons does barium have The barium atom has a total of 56 electrons because its atomic number is 56 and it is a neutral atom . [2] Now we have to fill these 56 electrons in the atomic .
How many valence electrons does Barium have? Barium is a significant chemical element and stands out uniquely just like any other element. The element is significantly useful in the commercial industry. .Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, .
The diagram below shows the number of valence electrons (VE) for the main group elements. A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = .How many valence electrons are found in the ground state electron configuration for Element 114? Answer: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 2 ; 4 valence electrons (from 7s and 7p orbitals. Also, elemenet 114 is in Group 4A, so it will have the same number of valence electrons as . Explanation: Barium is a heavy alkaline earth metal, and is the fifth row congener of beryllium, magnesium, calcium, and strontium. It has surprisingly little industrial importance according to this link. Barium is an alkaline earth metal; i.e. Group II of the Periodic table. Therefore, it has 2 valence electrons. Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .

An atom of the element barium (Ba) has two valence electrons. Barium is in the 6th period of the periodic table and is part of the alkaline metal.00Sunday, August 04, 2019Edit this post. A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Ok but how many valence electrons does an atom of Barium have? In the case of Barium the valence electrons is 2. Now let's check the facts about Barium.
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable .
How many valence electrons would an element with atomic number 56 have? The element with atomic number 56 is barium, a group 2 alkaline earth metal. Its atoms have two valence electrons in the 6s .The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 3d 10 4p 1, which contains three valence electrons (underlined). The completely filled d orbitals count as core, not valence, electrons. Transition elements or transition metals.
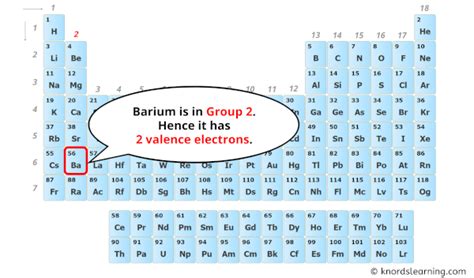
Ba: properties of free atoms. Barium atoms have 56 electrons and the shell structure is 2.8.18.18.8.2. The ground state electron configuration of ground state gaseous neutral barium is [ Xe ]. . As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into . sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . Method 1: From the Periodic Table. To find out the valence electrons of Barium, you have to see the position of barium in the periodic table. More specifically, you have to see the group wise position of Barium element in the periodic table. From the above image, you can see that the Barium (Ba) is present in the group 2 of periodic table.
Exercise 7.3.13 7.3. 13. Hund's rule states that the most stable arrangement of electrons (for a ground state electron configuration) has a filled valence shell of electrons. has three electrons per orbital, each with identical spins. has values greater than or equal to +1. has the maximum number of unpaired electrons, all with the same spin.
The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what order the positions are used.) For example, the Lewis electron dot symbol for calcium is simply. Figure 1 shows the Lewis symbols for .Strontium-90, a radioactive isotope, is a by-product of nuclear reactors and present in nuclear fallout. It has a half-life of 28 years. It is absorbed by bone tissue instead of calcium and can destroy bone marrow and cause cancer. However, it is also useful as it is one of the best high-energy beta-emitters known.
See scores, popularity and other stats for the anime Saimin Seishidou on MyAnimeList, the internet's largest anime database. Hajime Tanaka possesses powers quite unlike that of other students. Thanks to his hypnotic abilities, he can impose his will upon any individual he comes in contact with. He uses this skill to convince a .
how many valence electrons does barium have|Barium